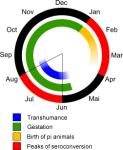
The Influence of transhumance on seroconversion and birth of PI animals
Within the framework of a study on the influence of BVD on the fertility of Swiss dairy cows it was discovered that the seroconversion rate in animals monitored peaked between June and August, as well as between January and April. Transhumance during summer explains the summer peak. As at that time the majority of heifers are in their early pregnancy a great number of PI animals are born next winter [26].
A. Transmission between individuals
Persistently infected animals clearly take pride of place in the epidemiological processes. Throughout their lives they permanently shed virus in large quantities via secretions and excrements. Acutely infected heifers can also serve as a carrier. However, they shed virus in considerably smaller quantities and also only during a few days. The Border Disease Virus of sheep can be transmitted to bovines, similarly the BVD Virus can be transmitted from bovines to ovines (sheep) and - in extremely rare cases - to caprines (goats). Many wild ruminants can contract BVD, however, it is unlikely that they have a reservoir function.
Direct transmission
- The virus is taken up perorally and/or nasopharyngeally. Under natural conditions, direct transmission, e.g. via mouth to mouth contact, is most effective. As little as a one-hour contact may be sufficient for transmission [60].
- Semen: BVDV can also be transmitted via semen by persistently infected as well as acutely infected bulls. The infection rate can be 100% in the case of PI bulls (AI) [61], but virus titres in acutely infected bulls are lower [62].
- In principle transmission during embryo transfer is possible, it can, however, be avoided by adequate hygienic measures.
Indirect transmission
- Rectal examination: A study published in 1994 describes a rectal examination of 8 heifers by use of a glove contaminated by a PI animal. All 8 animals were infected and had an acute infection. In the case of 5 animals virus was detected in addition to seroconversion. Two control animals, which had not been examined rectally, were shown to have neither seroconversion nor virus [63]. This study demonstrates that BVD can be quite easily transmitted in such a fashion. Pregnancy examinations, which mainly take place prior to attainment of foetal immunocompetence, are particularly risky.
- Contaminated hypodermic needles, nose tongs [64]
- Non-sterile vaccination techniques, contaminated pens: Following the contamination of a rubber membrane of a vaccination container that was then pierced twice with a hypodermic needle two calves were infected with BVD. A stable, which immediately before had been occupied by a PI animal and then re-occupied with 3 seronegative calves led to the infection of two of these calves. Two seronegative calves that were moved into a stable occupied by a PI animal four days previously were not infected [65].
- Contaminated live vaccines: 1999 saw a BVD outbreak in the Netherlands and, to a lesser extent, in Italy, following vaccination of animals with a BHV-1 marker vaccine. After the event the relevant batch was found to contain BVD viruses of genotype 2. Bovine calf serum is used for growing the vaccine in cell cultures. This supplies certain substances used for cell growth (e.g. lipids) and contains hormones and growth factors. In 1991, 49% of 190 tested (commercially available) BCS lots were virus positive [66]. Although controls have been vastly improved since then, a contamination of vaccines – as confirmed by the above example – can never be totally excluded.
- Arthropods: Experimental transmission by blood-sucking flies (S. calcitrans, H. pluvialis) has been described [67].
- Aerogenic transmission has been mentioned by a few authors [65][68][69], to date, however, this remains controversial.
B. Transmission between herds
- Animal markets, exhibitions, common pastures, transhumance: Direct contact (immediate as well as by proxy, e.g. via personnel) permits a circulating virus to easily be transmitted from one herd to another. Transhumance plays an important role in the epidemiology of BVD infection in Switzerland [70]. Pregnant heifers graze in the alps together with animals from other farms. If they are infected during the critical period of gestation the foetus is at risk of being infected – with the well-known consequences. As with a greater number of animals the likelihood of one or several PI animals being among them rises, the risk of future persistently infected virus carriers being generated also increases. These will be born during winter on their farms of origin (see picture on the right). By way of a preventive measure the animals could be vaccinated against BVD prior to giving birth – only it is doubtful whether the BVD vaccines used at present are effective (also see “Vaccination: the problem with BVD”).
- The uncontrolled acquisition of calves for meat production is risky for two reasons, first because those calves are frequently born of heifers that had been inseminated by bulls for meat production and had also exposed to a higher risk of infection during transhumance and, second because most of the poor growth calves go into meat production anyway. The theoretical risk of importing a PI animal when buying 20 uncontrolled calves amounts to around 33% (at a PI prevalence of 2%) [54].
- Acquisition of pregnant animals whose calf is persistently infected